From Photographer to Naturalist
“Where is all this wild life you show so many pictures of and talk so much about?” is a question repeatedly asked us after an evening program at the Rim.
The answer, to a naturalist, is simple. Direct him to the habitat, and he knows the rest. To the layman, you must all too often begin as far back as the definition of the word “habitat”, and go on from there in an effort to condense in a verbal moment information which actually has taken years to compile into volume after volume of painstaking research, observation and experience, and which is still incomplete.
In the beginning, the amateur photographer of wild life is content to ask someone else for the answer to his questions. But inevitably the fascination of his pursuits make him want to find the answers for himself. It goes something like this:
One day going up a mountain trail that cut through a steep talus slope which ended in a lush green meadow a few yards below the trail, our novice caught a flash of movement among the boulders below him. Readying his camera, he watched and waited and was soon rewarded by the appearance of a strange little rabbit-like animal about six or eight inches long, dragging a sprig of Cascade aster through the rocks. The little creature paused just long enough for one click of the camera and disappeared.
The developed picture revealed that while the little animal was rabbit-like, there were significant differences. His ears were short and round. He seemed to have no tail at all, and our photographer recalled a peculiar high squeaking call which he could not associate with rabbits.
A naturalist friend glanced casually at the picture and remarked, “So you have been up in the talus slopes.”
Our friend was surprised. “How do you know that?”
“That’s where this fellow lives”, was the answer. “This is the cony, or pika, sometimes known as the little chief hare, the haymaker, or the ventriloquist of the talus slopes.”
“Do you mean,” he asked incredulously, “that you never find this cony or whatever you call him any place else?”
“Not precisely,” said the naturalist, “but that is where you are most likely to find him, because such terrain affords him the most of what he needs for existence – – the green grasses, sedges and flowering plant for the little hay stacks which he puts up under rock slides during the summer for his winter food supply, the quick shelter among the rocky crevices both from the weather and his natural enemies.”
“Well, then,” said the photographer, with a gleam in his eye, “if I want more pictures of the cony or the pika, all I have to do is find a rock slide and I can get all I want.”
“Not quite so simple as that. There must be food near the rock slide, and it must be at a certain altitude, usually from 5000 to 13,000 feet, depending on the section of the country he is in.” He turned to his library. “Look him up. You can find out all about him.”
He extended a book, but his friend laughed. “I’m not taking it as seriously as that. I know where to find him and that’s all I need to know.”
The naturalist smiled. “Well, I guess that settles Ochotona princeps.”
“What?” asked the photographer.
“His scientific name.”
The photographer laughed. “Oh,” he said, “That stuff is not for me.”
That evening the photographer showed his pictures to a group of friends, and when he came to his new little animal there was much excitement. He felt well prepared to answer any questions.
“What is it?” asked one. “It’s a – – – ” and he had to think a minute, but finally it came to him, “cony or pika” he said triumphantly.
Then someone said, “But what’s it’s scientific name?” This question came from a friend who was known as “The Scientist.”
“I don’t know,” he said. “I don’t care about scientific names.” And he went on quickly. “He lives in rock slides because that’s where his food is. I mean,” he said, as The Scientist raised his brows, “If the rock slide is close to a meadow, that’s where his food is, and also he can find protection from his natural enemies there.” He was beginning to sweat a little.
“Natural enemies?” inquired The Scientist. “What are his natural enemies?”
Our photographer was suddenly desperate.
“I haven’t looked that up yet,” he snapped, and suddenly he had brilliant flash of recollection.
“His scientific name,” he said “is Ochotona.”
“Oh, of course – – Ochotona,” said The Scientist, “but is it Ochotona princeps orOchotona shisticeps shisticeps or Ochotona brunnescens or Ochotona fumosa?”
The photographer wasn’t sure what happened after that.
Late that night he decided what he was going to do, and day-break found him headed for the home of his naturalist friend to borrow the book that told about the strange little animal that looked like a rabbit but wasn’t.
“That book,” he said, as casually as he could make it. “Could I–”
“Of course,” said the naturalist, and gave it to him at once. He hadn’t even put it back on the shelf. “While you are about it,” he added, smiling, “you will want to look up the golden-mantled ground squirrel, the yellow – bellied marmot, the pine marten and the red fox. There are many others but don’t try to take in too much at one time. ”
 The ground squirrel . . . |
The photographer gulped.
“I’ve marked the pages,” the naturalist said soothingly. “You see, one thing follows another.
We must know their habits, you see. Where they live, and how and when. Some are out in the day time, some at night. Some live in trees, others on the ground. Some hibernate, others do not, some migrate, others–”
The photographer stood up with an air of sudden decision, and started out.
“Wait,” cried the naturalist, “you’re forgetting to take my book!”
The photographer turned and for the first time that day he was smiling.
“I’ve got a better idea,” he said, “I’m going to buy one for myself!”
 and the marmot . . . |
 like the cony . . . |
 live where they do . . . |
 because of what grows there. They eat the vegetation . . . |
 and the marten . . . |
 and the fox eat them. |
The Crater Lake Community
Probably the most asked question concerning Crater Lake is “Are there any fish in the lake?” To answer “yes” is easy. Such a reply, however, calls to mind the question of what they use for food. This, in turn, leads to a discussion of conditions which make it possible for fish to live and maintain themselves in Crater Lake.
Crater Lake rests in an unusual setting in comparison with most bodies of fresh water. It lies in the top of an ancient mountain – – old as man reckons time, but geologically recent – – the upper 5000 feet of which was destroyed about 6450 years ago. This destruction was in nature of a collapse which dropped the top of the mountain into a great void within its lower reaches and produced a cauldron – – or “caldera,” in the geologist’s terminology – – nearly 4000 feet deep and from four to six miles in diameter.
Preceding the collapse, a brief series of gigantic pumice eruptions withdrew great amounts of material from below the mountaintop and contributed to formation of the mammoth chamber. Some of these outpourings rushed down the slopes as flaming avalanches of gas-charged lava, each pushed along by its own jet-propelled impetus. Aside from filling stream and glacier-cut valleys, they engulfed and destroyed the forests and all other life, effectively sterilizing the area for miles around. Incidentally, the engulfed forests, through the medium of radioactive carbon, give us our best evidence as to the date of these last eruptions.
Since the collapse, precipitation has filled the cavity to a depth of nearly 2000 feet with water of great clarity and pureness. Its salt content is less than one-sixth that permitted for drinking purposes. Its bacteria count, even in parts where recent storms had carried large quantities of sediment into the lake, was found to be exceedingly low. The shores and bottom are rocky and the lake bed drops rapidly into deep water. There is no true shallow zone or real emergent vegetation. This geologically young lake – – probably less than a thousand years old at its present level – – has no beach worthy of the name.
The unusual setting of Crater Lake in the top of a mountain isolates it from the ordinary channels through which living things migrate and extend their ranges. This old volcanic cone slopes away on all sides. There is no higher ground from which rivers flow into the lake and which could carry living organisms, although there are numerous small cascades whose origins are in melting snows higher within the walls. Neither are there known channeled outlets, which could also serve as pathways of migration. Compared with most lakes, which in reality are only widened streams, Crater Lake is separated from the usual sources of plant and animal population. The life which exists there had to come into the lake by extraordinary means- – the hard way. There was no readily accessible reservoir.
It is interesting to conjecture just how life did come to Crater Lake Briefly, many lower plants and animals pass into stages of existence which are resistant to drying, freezing, and other conditions inimical to normal active life. Frequently, such inactive stages are associated with reproductive processes and involve eggs or spores which can renew activity at some later time when conditions are right. The shallow vernal pond which appears each spring, blooms rapidly and abundantly with diverse plant and animal life, and completely dries up later each summer is an example of this phenomenon. Since many of the forms found in Crater Lake have such inactive stages, it is easy to understand how they came to be there. Bits of mud clinging to the feet of bird could have brought many of them. Some could even have been carried by wind. Others could have been introduced with planted fish.
The Crater Lake community is complex. While the number of forms is small compared with many lakes, among the animals are representatives of most groups found in other fresh waters. In considering the community, however, the green plant is the key to its existence. As is true elsewhere, the green plant with its almost magic chlorophyll supplies all the energy used by animals. It alone has the unique ability to trap energy from the sun and make it available to other living things. It does this by combining two simple substances, carbon dioxide and water, to form grape sugar which is rich in tied-up energy.
This energy is passed on in one form or another to animals and some other plants. The fish – – or man, who eats the fish – – thus derives its very existence, perhaps through a long line of progressively smaller animals, from the simple green plant which started the processes. The biologist calls this a food chain, with the green plant at one end and the large animal at the other. This is the sort of relationship which makes it possible for fish to live in Crater Lake.
Of the green plants in the lake the most important, and practically the only ones, are the algae. These simple, essentially one-celled plants exist singly or in small groups that ordinarily can be seen only with the aid of a microscope. Some are grouped into long thread-like filaments. The filaments in turn may be gathered into jelly-like balls or masses large enough to be seen with the naked eye.
It would be difficult to assign relative importance to the members of this large plant group. Surely those classed as blue-greens, having a blue pigment which partially masks the green of the chlorophyll, appear most abundantly. One of these, Nostoc, is found growing in ball-like masses attached to rocks and among mosses. Other blue-greens are Oscillatoria, Calothrix, and Chroococcus (Brode, 1938). Other important algae, however, are certain filamentous greens, for example, Mougeotia and Zygnema.Rocks and logs along the shore show a conspicuous growth of Cladophora (Brode, 1938) and Ulothrix. Diatoms comprise the third important algal group. They are so abundant in “Fumarole Bay,” on the western side of Wizard Island, that the glass cases which enclose the living portion of this one-celled plant have formed, as countless numbers have died and settled to the bottom, a thick floor of diatomaceous ooze. This, of course, does not exhaust the algae found in Crater Lake but is only representative.
Mosses also are represented in the waters of the lake. Near Wizard Island, Fontinalisand Drepanocladus form a very thick mat on the bottom at a depth of 394 feet (Hasler, 1938) – – an indication of the great clarity of the water, which permits light to penetrate to such a depth in sufficient quantity for these plants to carry on the essential process of food manufacture.
Fontinalis also occurs on the “Old Man of the Lake,” the only place it is found near the surface (Brode, 1938). During the summer of 1953, a collection of several moss specimens was made on the “Old Man of the Lake.” Recent examination of these specimens by Dr. Henry S. Conard, Grinnell College, Iowa, showed all the material to be Scleropodium obtusifolium (Hook.) Kindb. It would appear, therefore, that this specie. is now the most abundant, if not the only, moss in this unusual habitat. Appreciation is expressed to Dr. Conard for making this identification.
According to Brode (1938), the only flowering plant growing rooted in the lake is the water buttercup (Ranunculus aquatilis capillaceus), found at depths of five to fifteen feet.
These, then, are the green plants which form the base of the great pyramid at the apex of which are the fish which dominate the waters.
Green plants alone, however, would not support a population of rainbow trout or sockeye salmon. These animals require a “meat” diet. Between them and the plants there is of necessity at least one intermediate animal which feeds upon plants and serves to convert plant materials into animal substance. Several inhabitants of Crater Lake serve in this capacity. Some feed directly upon the living plants while others function as scavengers which utilize dead organic matter for food. Hubbard (1934) lists five such converters. The most important is the “bloodworm, ” or midge larva, which feeds almost exclusively upon algae. While some larvae are taken by fish, the pupal stage, since it is less active, appears to be a more important component of the fish diet. Caddis fly pupae are also found in some abundance in fish stomachs.
Snails, which feed on diatoms as well as upon dead animal matter are often taken by fish. Chief Ranger L. W. Hallock reported the catch of a 23-inch rainbow, the major food item of which was the snail. This returning of dead animal matter directly to living flesh is an important short cut in the food cycle.
Certain small relatives of the crayfish which live in the lake also play their part in food conversion. Daphnia, the so-called “water flea,” is very tiny–it measures perhaps 2mm., 1/12 inch, in length–but exists in rather large numbers. Kemmerer et al. (1923) state that they are found mostly between about 250 and 300 feet or more below the surface. The preferred food item of these small crustaceans is the diatom. They, in turn, are of primary importance to the fingerling fish and often make up a considerable portion of the food of larger fish. Brode (1938) reported 7500 in the stomach of one fish. The freshwater shrimp, Hyalella, also forms part of their diet. The copepod,Cyclops, though not as abundant as Daphnia, plays a similar role as a converter.
Many of the smaller converters are also fed upon by large carnivorous water insects, which then fall prey to fish. Of these intermediate forms, dragonfly nymphs play an important part. While they themselves are seldom taken by fish, adults, as they fly over the water, often are caught by them. Adult whirligig beetles, which live in the water, also are important intermediates.
During the summer months, those fish which feed at the surface take advantage of any food items that may come their way. While no considerable number of fish stomachs were examined this season, those studied– from fish taken exclusively by casting from shore–had fed predominantly upon terrestrial insects. These consisted of various flies, bees, ichneumons, a great many long- horned beetles, butterflies and dragonflies. A few spiders were also found. Strictly aquatic forms were few and were primarily midge larvae and pupae.
Most of the fish reported were taken by Ranger Joseph C. Hunt. These were largely rainbow trout (Salmo gairdnerii irideus), although a few were sockeye salmon (Oncorhyncus nerka kennerlyi). The former ranged in length from about 12 to 22 inches, while the sockeyes had a maximum of about 12 inches. The fish, therefore, is an opportunist and takes advantage of what may come his way. In this manner he is able to encroach upon food supplies from outside the lake.
Other forms of animal life not of great importance as fish food are also found in Crater Lake. The endemic salamander, the Mazama newt (Triturus granulosus mazamae), occasionally furnishes a meal for a fish. This near relative of the frog, in company with the long-toed salamander (Ambystoma macrodactylum), lives under rocks along the shore.
Water-dwelling annelids, related to the common earthworm, are found in small numbers and sometimes are retrieved from fish stomachs. In a rather cursory examination of the aquatic community on the “Old Man of the Lake,” the remains of a hemlock tree with part of its root system below, and about five feet of stump extending vertically above, the water surface and which is carried about the lake by wind currents, there were found two kinds of these annelids. Among other interesting forms in this community were a large number of mites–relatives of the spider. Quite abundant also, were copepods belonging to the harpacticid group. Specific identification has not yet been made. The available literature indicates, however, that this group of microcrustaceans has not been previously reported from Crater Lake.
Fish, as previously stated, are the dominant forms of animal life in the lake. It should be evident that these inhabitants did not enter the lake by natural means. The first planting was made September 1, 1888, by William Gladstone Steel when he released 37 of an estimated 600 rainbow trout “minnows” with which he had started from the Gordon Ranch, 41 miles from the lake. The first trout was caught in 1901. In 1902, and in many years thereafter, other plantings were made by the National Park Service. A number of species were tried but, with plantings discontinued in the early 1940’s, the only ones that have persisted and reproduced are the rainbow trout and sockeye salmon.
The Crater Lake community is thus seen, from this brief review, to be a relatively closed community, at present essentially self-sustaining.
Some forms have been introduced artificially. Most of the inhabitants, however, have found their own ways there and have become established as important components which make their own peculiar contributions toward the total economy.
References
Brode, J. Stanley. 1938. The denizens of Crater Lake. Northwest Sci. 12 (3):50-57.
Hasler, Arthur D. 1938. Fish biology and limnology of Crater Lake, Oregon. Jour. Wildlife Management 2(3):94-103.
Hubbard, C. Andresen. 1934. Fact and fancy about Crater Lake fish. Report submitted to the Research Branch of the National Park Service, March 1, 1934. (MS. in Crater Lake National Park Library).
Kemmerer, George, J.F. Bovard and W. T. Boorman. 1923. Northeastern lakes of the United States: biological and chemical studies with reference to possibilities in production of fish. Bull. Bur Fisheries 39:51-140.

Crater Lake Bears. From kodachrome by Welles and Welles.
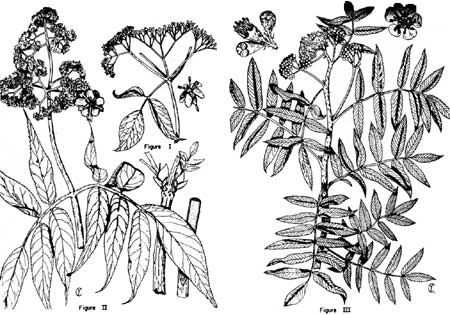
Three Similar Shrubs in Crater Lake National Park
By Charles F. Yocom
Visitors in Crater Lake National Park during the latter part of the summer frequently notice the attractive white blooms of certain green shrubs along the Klamath Falls entrance road (south entrance) and along the walks near the Information Building and the Lodge. Ranger naturalists are occasionally asked the name of one of these plants but from the description are usually unable to tell the visitor the exact name of the plant, for there are three different kinds of shrubs in the park that have somewhat similar appearing blooms and leaves. These are blueberry elder, Sambucus coerulea(Fig. I), Pacific red elder, Sambucus racemosa var. callicarpa. (Fig. II), and western mountain ash, Sorbus sitchensis (Fig. III).
Two of these plants, the elderberries, are in the same genus and the leaves and stems look nearly identical. They differ, however, in the shapes of the flower clusters. The blueberry elder has a cluster of flowers that appears somewhat similar in shape to an umbrella, whereas the white flower clusters on the red elder are dome-shaped or somewhat pyramid-shaped. If the branches of either are broken one can see that the stems are filled with a soft substance called pith.
Mountain ash is related neither to the elderberries nor to the ash tree, which it also resembles; but it is closely allied with the wild rose, apple, peach, pear, plum, chokecherry and serviceberry, all of which belong to the rose family. In some areas, mountain ash attains the proportions of a small tree; in Crater Lake National Park, however, it usually occurs as shrubs. The elders belong to the honey-suckle family and should not be confused with alders.
For structural differences between the three shrubs, see the table below. Blueberry elder occurs only along the south boundary of the park and is common along the roadsides between Klamath Falls and Fort Klamath. Mountain ash and red elder occur at higher elevations in the park and often are growing side by side. All of these shrubs furnish an important source of food for many birds and some mammals.
In late summer and fall visitors observe the attractive berries of these shrubs along the roads and walks. There is no difficulty in telling the two elders apart at this time of year, for they have berries colored according to their common names. Of course, those with blue berries (Sambucus coerulea) are found at lower elevations and are often sought for pies and wine. Mountain ash and red elder have berries that look somewhat alike, so one must look for differences in leaves and stems.
Also remember that the cluster of berries of mountain ash is somewhat umbrella-like in shape and that the berry cluster of red elder is oblong, the berries themselves being brighter red.
Comparative Table of the Three Confusing Shrubs
| Character | Mountain Ash | Red Elder | Blueberry Elder |
|
|
|||
| Color of flowers | white | white | white |
| Shape of flower cluster | umbrella-like | dome-shaped or pyramid | umbrella-like |
| No. of petals | 5 | 5 | 5 |
| No. of stamens | more than 5 | 5 | 5 |
| Color of berry | Orange-red | bright red | blue |
| Shape of berry clusters | flat topped cluster | oblong cluster | flat topped cluster |
| No. of leaflets | 9 to 11 | 3 to 9 | 3 to 9 |
| Color of leaflets | shiny green | dull green | dull green |
| Amount of pith in stem | very little | much | much |
Nesting Birds
During the summer, at least several visitors came into the Information Building to inquire about the two blue birds seen near the back of the building. Of those who saw the pair of mountain bluebirds, Sialia currucoides (Bechstein), only a few realized that the birds were nesting behind a half-closed window shutter on the second floor. When the nest, made up of grass, dead staghorn lichen and one piece of twine, was first discovered on July 11, it contained two eggs.
On subsequent visits the female was nearly always seen on the nest, incubating her undersized clutch of eggs. On July 21, two helpless, pink mites were seen for the first time. The babies grew rapidly; 20 days later one was still in the nest but the other had ventured as far as the ledge a foot away. When the nest was visited the following day, both young bluebirds had departed.
Other nests were found during the summer, most of them being located by observing the adults carrying food. In this way, a second mountain bluebird’s nest was discovered in a hole 20 feet up in a mountain hemlock, several hundred feet east of the Lodge.
The nesting hole of a red-breasted nuthatch, Sitta canadensis Linnaeus, also in mountain hemlock, was watched by numerous members of the morning Garfield Peak field trips. The dead stub, to which the nuthatches came regularly with food, was conveniently located near the first lookout along the Garfield Trail.
Mountain chickadees, Parus gambeli Ridgway, were found in a snag at Cold Spring Campground, and a pair of violet-green swallows, Tachycineta thalassina (Swainson), evidently reared a family in a cavity in one of the Wheeler Creek pinnacles. The swallows were observed making frequent trips to a small hole in one of the tall, spire-shaped formations, presumably feeding their young.
The nests of two Oregon juncos, Junco oreganus (Townsend), were discovered quite by accident when, in each case, the incubating female was flushed from her nest, well-hidden in a depression in the ground. One nest contained three eggs, the other held four; both were located between the highway and lower Munson Meadow. Unfortunately, there was not an opportunity to observe the hatching and growth of the young. Perhaps our visits were too frequent, perhaps some catastrophe overtook the females. At any rate, the nests were abandoned and neither adult was seen again in the immediate vicinity.
References
Farner, Donald S. 1952. The Birds of Crater Lake National Park. University of Kansas Press. ix, 190 pp.
Farner, Donald S. 1952. The use of the Wheeler Creek Pinnacles by nesting birds. Crater Lake Nature Notes 18:9-10.
Other pages in this section