Chemical Evidence of Thermal Components in Crater Lake Water
Elevated concentrations of boron and lithium are typically found in thermal waters of volcanic origin (e.g., White and others, 1976, Ellis and Mahon, 1977, p. 58-116). Because Crater Lake water is enriched in boron and lithium compared to local meteoric water and because the concentration of boron and lithium is either at or below the detection limit for these dissolved constituents in the cold spring waters, we evaporated water from 8 cold spring and 2 lake water profiles, collected at 100 m intervals, from 1 -liter to approximately 50 mL. This reduced volume was then analyzed for boron and lithium. The concentration of lithium and boron were then significantly above the detection limits and are reported in table 4.
The concentrations of boron (tables 1 and 4) in Crater Lake water is at least twice that of the cold-spring water, and the lithium concentration is at least 10 times that of the cold springs. If the chloride, boron, and lithium are derived from a thermal source, then the Cl/B and Cl/Li weight ratios would be expected to be similar to ratios from known hot springs in volcanic areas (table 4). Unfortunately, the cold water Cl/B ratios range from 6 to 33 and the lake water ratios range from 17 to 31. This overlap invalidates the use of the Cl/B ratio for identifying thermal components in the lake waters.
The Cl/Li ratio appears to be more diagnostic. In cold-spring waters the Cl/Li ranges from 540 to 4600, and in Crater Lake the ratio ranges from 220 to 280 (mean = 242, std. dev. = 20)(table 4). The Cl/Li weight ratio is substantially lower in lake water than in cold-spring water. Typical Cl/Li weight ratios for thermal waters from other volcanic areas range from 80 to 410 (mean = 246, std. dev. = 115)(table 4). The Crater Lake Cl/Li weight ratios are near the mean Cl/Li ratios for a variety of volcanic settings in the western United States. This also suggests that the additional chloride may be contributed by a thermal water.
Of the other anionic indicators of thermal waters, S04 and HCO3, S04 can arise from biogenic oxidation of sulfur and sulfides (Schoen, 1969; Schoen and Rye, 1970; and Brock and Mosser, 1975), and atmospheric C02 can also dissolve in the lake. We do not have the requisite isotopic data to determine the fraction of HCO3 and S04 contributed by this deep thermal fluid.
Crater Lake is a slightly alkaline (pH-7.5) sodium chloride-sulfate lake. This observation negates the possibility that acidic fumarolic gases such as HCl and H2S are being discharged into the lake bottom as was suggested by Van Denburgh (1968). If HC1 were being added to the lake, then the ionization of the HCI would make the lake acidic (pH<7). The oxidation of H2S, which generates sulfuric acid, also would tend to make the lake acidic. Thus, Na+ and Cl- appear to enter the lake together, probably dissolved in water. NaCl is not transported in a low temperature (t(150’C), low pressure (P<15 bars) gas. Additionally, the excess SiO2 discussed earlier suggests transport of SiO2 in water because little SiO2 is transported in a vapor phase.
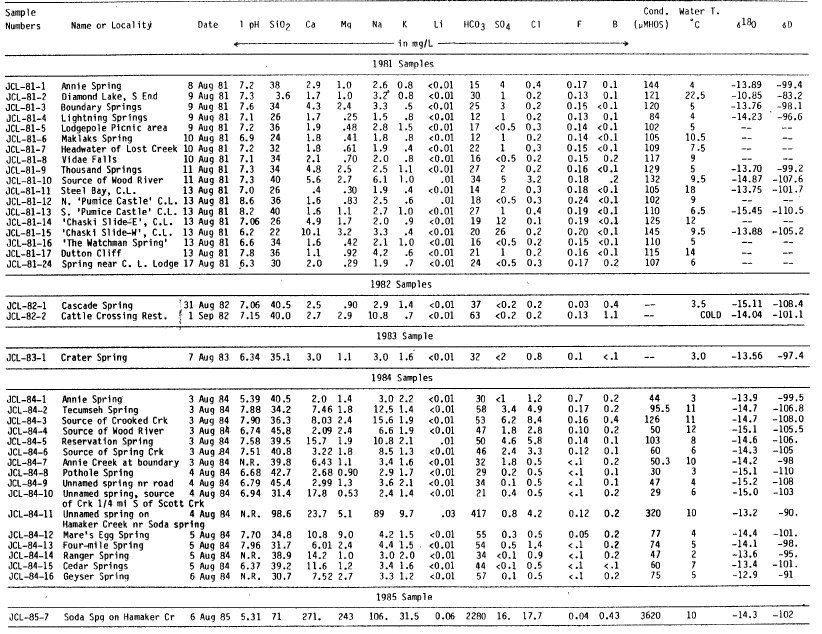 |
| Table la. Chemical Analyses of Springs in the Vicinity of Mount Mazama |
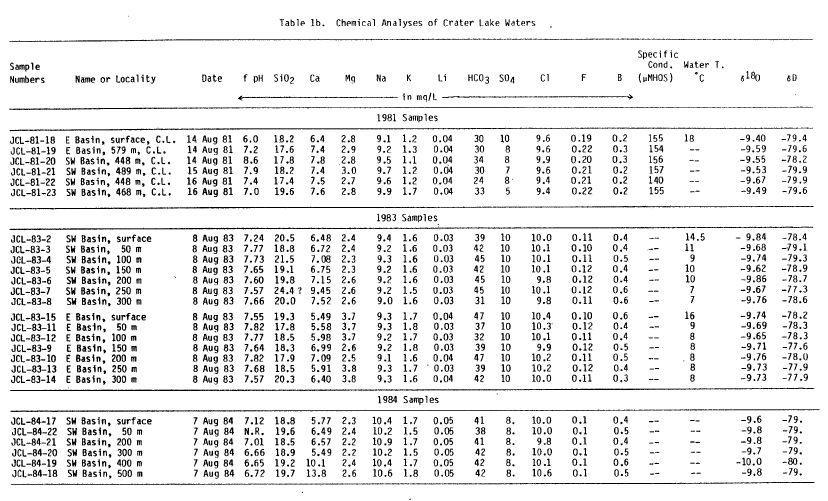 |
| Table 1b. |
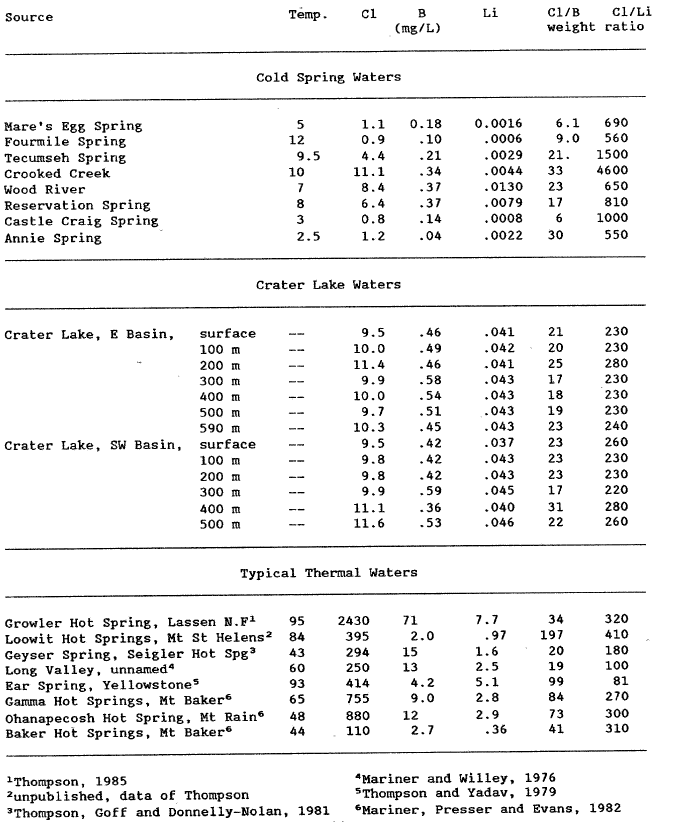 |
| Table 4. Analyses of B and Li in partially evaporated samples of lake water collected in 1985, recalculated to original concentrations, and values for other western U. S. Hot Springs |


