Laboratory Analyses
Silica was analyzed at 640 nm by a modification of the molybdenum blue spectrophotometric procedure described by Shapiro and Brannock (1956) using 10 mL of the filtered acidified spring water.
Boron was determined spectrophotometrically using the carmin procedure at 600 nm (Brown and others, 1970).
Bicarbonate was determined titrimetrically as alkalinity using a constant-drive buret, a combination pH glass electrode, a specific ion – pH meter, a strip chart recorder,- and standardized sulfuric acid (0.05N). The laboratory pH was taken as the pH at the start of the alkalinity titration. If bicarbonate was analyzed in the field, the analysis was not repeated in the laboratory.
Sulfate was determined by a turbidimetric procedure using BaCl2 to precipitate BaSO4. The 1983 samples of lake water were determined by ion chromatography (Fishman and Pyen, 1979).
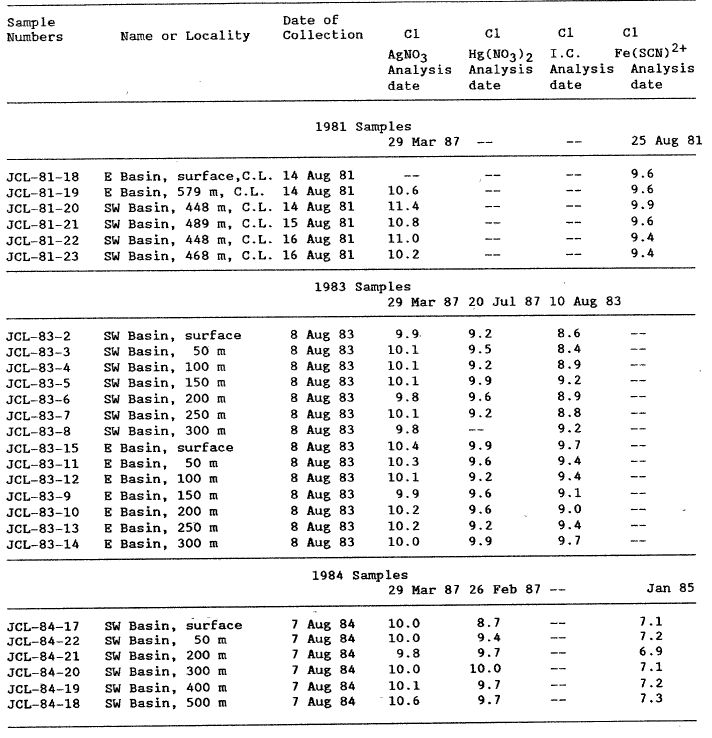
Chloride was determined by four procedures: a) the colorimetric ferric thiocyanate method (Fishman and Friedman, 1985); b) the manual mercurimetric titration procedure (Brown and others, 1970), c) an ion chromatographic procedure using a HCO3 – Co3 eluent and conductivity detection (Dionex model 16), and d) an automated AgNO3 titration (Brinkman, model 682). Results of the various chloride analyses are reported in table 2.
Fluoride was determined by an Orion ion specific electrode; TISAB II was mixed 1:1 with all samples and standards. The 1983 samples were analyzed by ion chromatography (Fishman and Pyen, 1979).
Sodium and lithium were determined simultaneously by flame emission spectroscopy (FES) in a fuel-rich, air-acetylene flame with added potassium ion (0.1 percent v/v) at 589.0 nm and 670.8 nm, respectively.
Potassium was determined by FES in a stoichiometric air-acetylene flame with added cesium ion (0.1 percent v/v) at 766.6 nm.
Calcium and magnesium were determined simultaneously by atomic absorption spectroscopy (AAS) in a stoichiometric air-acetylene flame with added La(III) (1.0 percent v/v) at 422.7 and 285.2 nm, respectively.
Specific Conductance was determined following the procedure described in Brown and others (1970).
Deuterium analyses were made following the procedure of Bigeleisen and others (1952).
Oxygen-18 analyses were made following the procedure of Epstein and Mayeda (1953).
Brand names used are for information purposes only and do not constitute a recommendation by the U.S. Geological Survey.
 |
|
Table 2. Chloride Analyses of Crater Lake Waters (Cone. in mg/L) |


