Hydrology of Crater, East and Davis Lakes, Oregon by Kenneth N. Phillips
CHEMISTRY OF THE LAKES
By A. S. VAN DENBURGH
CRATER LAKE
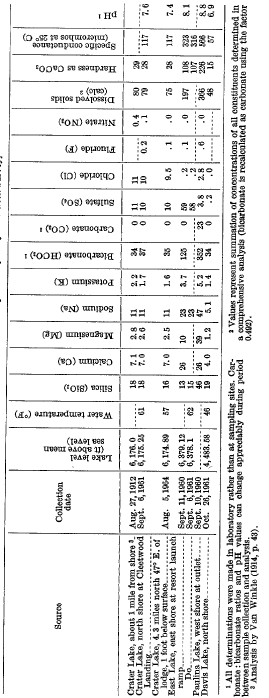
Crater Lake is fed almost wholly by direct precipitation, and the chemical character of the lake reflects the dilute nature of that supply. The dissolved-solids concentration is about 80 ppm, made up mostly of silica, calcium, sodium, and bicarbonate (table 13). However, Crater Lake also contains significant concentrations of sulfate and chloride (about 10 ppm of each) not usually found in the surface water of humid mountainous regions.
The lake water appears to be practically uniform in chemical quality both areally and vertically. Partial analysis of 12 samples taken August 5, 1964, at three sites and at depths ranging from 1 foot to 1,900 feet showed silica concentration that ranged from 16 to 17 ppm, chloride from 9.5 to 9.8 ppm, and specific conductance from 115 to 118 micromhos at 25° C.
Crater Lake contains about 1.5 million tons of dissolved solids within its 14-million-acre-foot water body. Through 1964, the lake has been sampled only three times-in 1912, 1961, and 1964-for comprehensive chemical analysis. The results (table 13) suggest that very little net change in dissolved-solids content or chemical character occurred during the 52-year period.
Direct precipitation accounts for the greatest increment of water added to the lake, but its dissolved-solids contribution is of only secondary importance. The bulk of dissolved material now present in the lake has undoubtedly been derived from three principal sources: (1) Spring discharge and runoff from the caldera sides above the water level, (2) dissolution or mineralogic alteration of slightly soluble components of the caldera wall below the lake surface, and (3) spring flow that may issue from the lake-basin sides and bottom below the present lake level. The relative importance of these sources is uncertain. However, three of the principal constituents of the lake water-silica, calcium, and sodium-axe the most mobile and common products of the low-temperature hydrochemical breakdown of most igneous rocks, including the group of volcanic rocks that form Mount Mazama.
The accumulation of sulfate and chloride in Crater Lake may not be due entirely to the normal processes of hydrochemical rock breakdown. The two constituents, and perhaps some silica and sodium as well, may have been contributed to the lake by thermal springs or fumaroles, probably located below the present lake level. Such springs and fumaroles are a common expression of hydrothermal activity at a site of volcanic eruptions.
This opinion is supported by the contrastingly small concentrations of the two constituents observed in water of Rogue River near Prospect, Oreg., about 25 miles southwest of the lake. This river drains an area adjacent to Crater Lake and underlain predominantly by volcanic rocks. Observed concentrations of sulfate and chloride were less than 2.0 ppm and usually less than 1.0 ppm in monthly samples collected during the period August 1960-September 1961.
Leakage plays an important part in the chemistry of Crater Lake. The estimated water loss (about 90 cfs) removes approximately 7,000 tons of dissolved solids from the lake each year, an amount that may be more than the quantity added annually. Thus, the lake may actually be freshening slightly over a long-term period. However, the lack of appreciable chemical change during the 52-year period 191264 indicates that the freshening, if it does occur, is a slow process.
| TABLE 13.-Chemical and physical character of the lakes |
 |
***previous*** — ***next***

